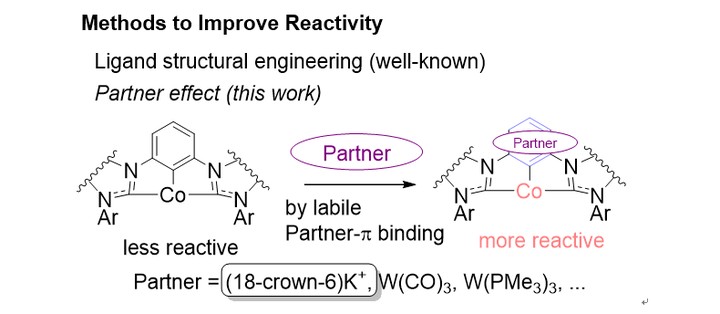
Abstract
A strategy to tune the catalytic behavior of a organometallic catalysts rather than ligand engineering is suggested in this work, by computationally studying the effect of (18-crown-6)K+, W(CO)3 and W(PMe3)3 on the reactivity of a Pincer-Co catalyzed nitrile hydroboration reaction through π-coordination to the ligand aromatic ring. These extra additives, as called by the partners, binds the central phenyl ring of the ligand by either dispersion or chemical bonding. The electron-richness of the cobalt center is tuned by the partner, and follows the order (18-crown-6)K+ > W(PMe3)3 > no partner > W(CO)3. While the influence of covalent W-containing partners parallels the electron-richness of W, the non-covalent partner, (18-crown-6)K+, surprisingly increases the donor ability of the Pincer ligand, through polarization effect. All the elementary steps involved in the nitrile hydroboration reaction are influenced by the partner, and the overall barrier is lowered by a surprisingly large extent of 4.9 kcal/mol in the presence of (18-crown-6)K+, suggesting a charming partner effect to be explored by experimentalists that the reactivity of a catalyst can be consecutively tuned without ligand modification.