The Potential Role of Addition Coupled Electron Transfer (ACET) in Single Atom Catalysis: The Hydrogen Transfer from Metalloporphyrin to Imine is an ACET
Abstract
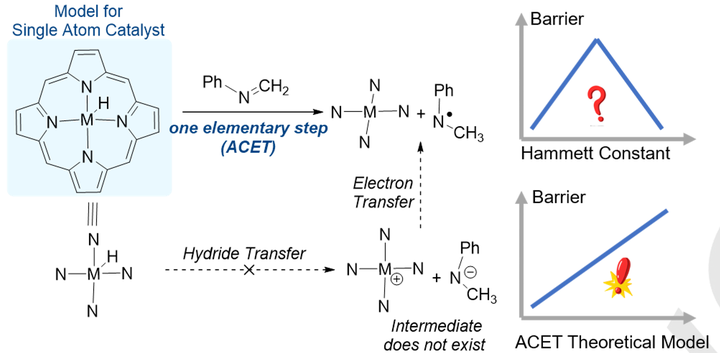
The formal hydrogen transfer from single atom catalyst to unsaturated compounds is of great interest in the catalysis research. With the hydrogen transfer from metalloporphyrin hydride (MPcH, M = Fe, Co) to imines as an example, we have shown that this reaction is an addition coupled electron transfer (ACET) reaction instead of a hydride transfer, by combining density functional theory (DFT), multireference calculations, intrinsic reaction coordinate analysis and substituent effect study. The ACET mechanism is universe in both low-polar solvent (dichloromethane) and high-polar protic solvent (2-propanal). The barrier versus Hammett substituent constant relationship under dichloromethane solvation features a volcano-like shape, in which both electron-withdrawing and electron-donating groups accelerates the reaction. While the structure-reactivity relationship cannot be rationalized by either substituent constant σp or the spin delocalization constant σJJ, it can be successfully explained by a theoretical model of ACET proposed by us for the first time in this work. This work shows that ACET may be ubiquitous in single atom catalyzed addition reactions.