Mechanistic Basis for Oxoammonium Salt-Mediated Tertiary Alcohol Oxidative Transformation Decoded by Computations and Experiments
Abstract
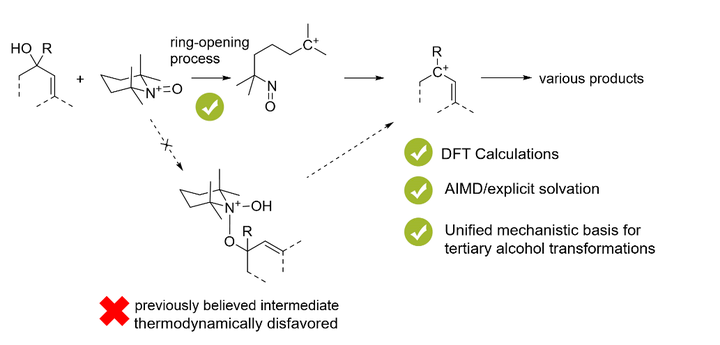
Oxoammonium salts have demonstrated superior efficacy as reagents in mediating various reactions involving tertiary alcohols, including the eliminative functionalization of tertiary benzylic alcohols, oxidative rearrangement of tertiary allylic alcohols, and oxidative Nazarov cyclization. These reactions have been believed to be triggered by the addition of alcohols to the N−O double bonds of oxoammonium cations. However, in this work, a combination of density functional theory (DFT) and ab initio molecular dynamics (AIMD) calculations shows that the formation of this adduct is thermodynamically unfavorable. A thorough exploration of the possible mechanisms has shown that the reaction occurs through the ring opening of the oxoammonium cation. The oxoammonium cation acted as a masked tertiary carbocation and triggered the subsequent reactions. The computational results were further supported by control experiments and high-resolution mass spectroscopy (HRMS) characterization.