Unveiling the Origin of Chemoselectivity of Bismacycles-Mediated C─H Arylation of Phenols: From Mechanism Concept to New Coupling Design
Abstract
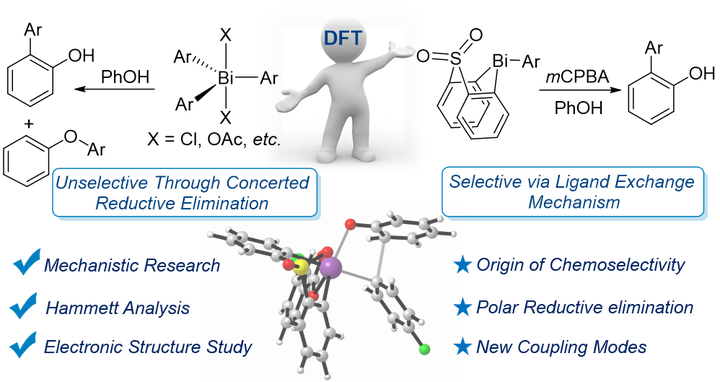
Recently, a bismacycles-catalyzed functionalization has been presented as an elegant strategy to perform C─H arylation of phenols by Ball and co-workers. By density functional theory calculations, we performed thorough study on the mechanism, selectivity and electronic nature. Firstly, the reaction is initiated by an oxidative addition (OA) of aryl bismacycle by mCPBA which occurs through a stepwise mechanism, in which a rate-determining oxo-transfer is followed by the barrierless addition of mCBA. The resulting hexacoordinated Bi(V) intermediate which subsequently undergoes a rapid ligand exchange (LE) with phenol, followed by a fast reductive elimination (RE) to give the C–C bond formation product. Secondly, by exploring chemoselectivity for C─O versus C─C arylation, we disclose that the C─O bond RE leading to diphenylether exhibits a significantly higher barrier than the o-arylated RE by 4.3 kcal/mol, in sharp contrast to the previously reported unselective coupling with BiPh3Cl2 by Barton. The difference in chemoselectivity originates from a switch in the RE mechanism according to whether a LE with phenol undergoes prior to the Bi(V)/Bi(III) RE step. Under Ball’s conditions the stronger basicity of the hydroxide ligand ensures a fast LE to give hexacoordinated Bi─OAr intermediate that strongly favours the C─C bond formation, whereas in Barton’s work the LE could not occur, and the RE has to follow a concerted intramolecular proton-transfer mechanism, of which the chemoselectivity is low (0.8 kcal/mol). Thirdly, the effect of substitute groups and then the nature of the RE are studied using Hammett analysis, atomic charge analysis, and designed reaction modes, which all revealing that the C─C RE from Bi(V) is polar in nature. Finally, new Bi(III)/Bi(V) cross-coupling modes such as aryl-enolate and alkene-enolate couplings are designed and shown substantially reduced RE barriers for C─C bond formation and the chemoselective preference is increased remarkably.