Abstract
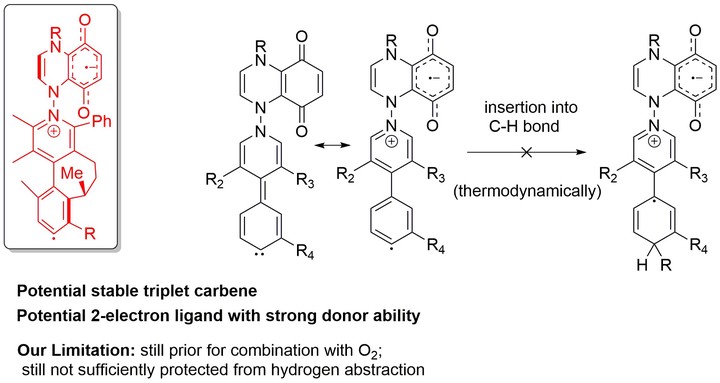
In sharp contrast to the widely studied and applied stable singlet carbenes, only several kinetically persistent triplet carbenes have been studied, and thermodynamically stable triplet carbenes are much less developed. With the Gibbs free energy of C-H bond insertion into methane as a probe, DFT calculations were employed to examine a variety of candidate molecules for stable triplet carbenes. Guided by these calculations, some molecules with significant stability against C-H insertion were designed by fine tuning of geometry and electronic structures. These compounds might be potential candidates for experimental development of stable triplet carbenes.
Type