A Mechanistic Study on Gold(I)-Catalyzed Cyclization of Propargylic Amide: Revealing the Impact of Expanded-Ring N-Heterocyclic Carbenes
Abstract
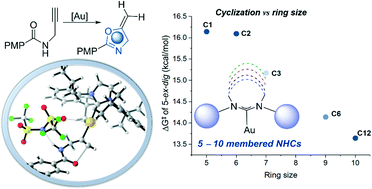
The interest in expanded-ring N-heterocyclic carbenes (ER-NHCs) has been recently given a noticeable attention, especially with the Au(I)-catalyzed activation of alkynes. Herein, we report density functional theory (DFT) investigations on the Au(I)-catalyzed cyclization of propargylic amides to exploit the mechanistic effect of variable ER-NHCs to shed some light for further future developments. Mechanistically, the reaction undergoes an intramolecular nucleophilic addition/cyclization after π-complexation with the alkyne miotey while counteranion (NTf2─) is interacting with amide group. Subsequently, N-deprotonation by the counteranion followed by C-protonation (protodeauration) process furnishes the cyclized product and regenerate the LAuNTf2 to continue the catalytic cycle. The cyclization becomes highly disfavored when the counteranion is absent. Both the cyclization and protodeauration steps favor the 5-exo over 6-endo product with unsubstituted terminal alkyne. The ring-size effect of the NHCs is explored, where NHCs larger than 5-membered ring provides intrinsically larger steric demanding with the same aryl group on it, which is shown to inhibit the reactivity. For NHCs with similar steric properties, ER-NHCs accelerates the cyclization step. Various electronic structure analysis shows that for the Au(I) center ER-NHCs are less effective electron donor because of less orbital overlap and render the Au(I) more electrophilic. This work provides new dimensions to the development of Au(I)-catalyzed methodologies to engineering ligands.